Xeomin
Xeomin (incobotulinumtoxinA), made by Merz Pharmaceuticals, is approved by the US FDA for the treatment of adults with cervical dystonia or blepharospasm. IncobotulinumtoxinA is made from the bacteria that causes botulism. Botulinum toxin blocks nerve activity in the muscles, causing a temporary reduction in muscle activity.
FDA approval of XEOMIN, was based on the results of two U.S. clinical trials. A study cited in the Journal of Neurological Sciences on the efficacy and safety of XEOMIN found it showed “non-inferiority” to Botox when used in the same doses to treat cervical dystonia. It concluded that XEOMIN is a safe and effective treatment for the disorder.
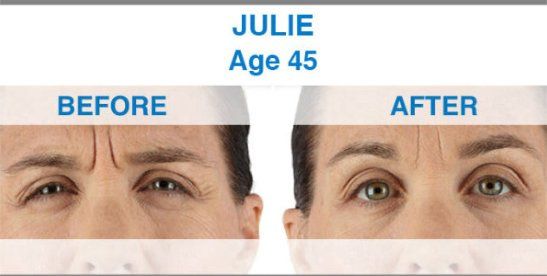
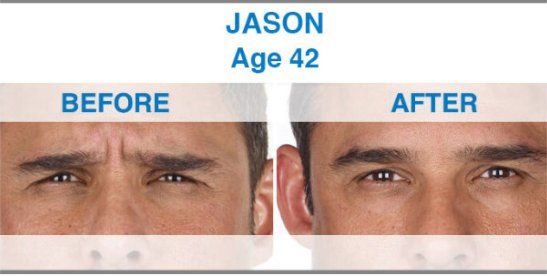
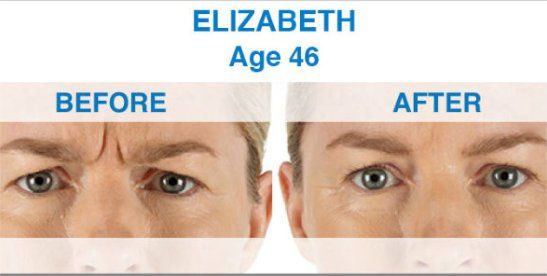
Xeomin is not yet approved for any cosmetic indications. By contrast, other drugs in this category, including Botox and Dysport, do have FDA approval for the treatment of facial wrinkles. Now that Xeomin is available and FDA approved, doctors can use it off-label to treat the same facial lines treated by Botox and Dysport, including frown lines, crow's feet and forehead wrinkles.
Worldwide, more than 84,000 people have been treated with Xeomin injections. The U.S. is actually the 20th country to approve this new drug. It will be available in 50-unit and 100-unit vials.
Better or worse then Botox?
Xeomin is the first one that does not need to be refrigerated before use, which may simplify distribution. Xeomin is also has no additives, just botulinum toxin type A. This may mean that there is less risk of developing antibodies against Xeomin than other available neurotoxins. The body develops antibodies in response to a foreign invader and attacks. In theory, antibodies could prevent a neurotoxin from having its desired effects. The effects of Xeomin occur within one week, and the results last from three to six months, making it comparable to Botox in terms of both onset and duration of action. Xeomin should not be used interchangeably with other botulinum products.
Worldwide, more than 84,000 people have been treated with Xeomin injections.
What are the risks?
There is a risk that all botulinum toxin products may spread from the area where they were injected to other parts of the body, causing potentially life-threatening swallowing and breathing problems. This was predominantly seen in children treated with Botox off-label for cerebral palsy. These issues have not been reported among people who received botulinum products for cosmetic uses or to treat blepharospasm.
Other risks may include bleeding and bruising at the injection site and allergic reactions such as itching, swelling or shortness of breath. Your doctor should discuss all the potential risks of this procedure with you during your consultation.
When Xeomin is used to treat cervical dystonia, side effects include neck pain, muscle weakness, injection site pain and musculoskeletal pain. When used to treat blepharospasm, the most common side effects of Xeomin were eyelid sagging, dry eye, dry mouth, diarrhea, headache, visual impairment, shortness of breath (dyspnea), and upper respiratory infections.
How much does Xeomin cost?
Xeomin cost is slightly lower than Botox. Call our office for Xeomin prices and specials of the month. 818-280-0700
How do I know if this procedure is right for me?
Simply call our office 818-280-0700 for a complimentary consultation appointment.
Questions About Xeomin inSimi Valley & Northridge?Request Your Complimentary Consultation Today!
Book Online
Call at
805-526-3365